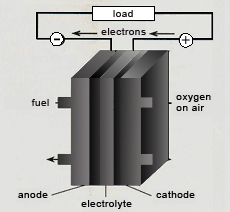
Fuel cells will play a key role in future energy supplies as an efficient, flexible in use and silent system. They produce electricity by means of a chemical and direct conversion of the fuel's energy without any thermic intermediate steps like in nowadays power plants.
Depending on the type fuel cells can run on hydrogen or methane (from natural gas, biogas or gas from sewage treatment plants), mixtures of both or liquid methanol. All fuels do not react directly with oxygen, but are being separated by an appropriate electrolyte (see figure). The electrolyte is only permeable for certain ions. Only when ions are exchanged over an external connection the reaction starts. The resulting electricity is the desired fuel cell process.
With hydrogen as a fuel there are no harmful emissions whatsoever, only steam as an "exhaust fume". The process in the fuel cell thereby represents a reverse electrolysis. Hydrogen ions combine with oxygen to form water or steam respectively, producing electricity and heat.
Hydrogen + Oxygen → electrical Energy + Water + Heat
In this process relatively high electrical efficiencies of 30% up to 55% can be gained depending on the fuel cell type. Fuels cells can be utilised for a wide range of tasks:
- for portable applications such as laptops, mobile phones or cameras where they replace the batteries
- for the automotive industry where they are regarded as the motor of the future due to their high efficiency especially in the partial load range. Nearly all car manufacturers push the development of fuel cells and mass production can be expected in the forthcoming decade
- for stationary use to supply homes efficiently with heat and electricity (CHP using fuel cells). Many of these small units will be connected to each other in the future to form a "virtual power plant".
Fuel cells can also be used in the high power range of industrial applications or conventional power plants, where they help renewables to integrate into the grid. For this, fuel cells will be used as part of re-generation plant, together with electrolysers and hydrogen storages.
Today mainly four fuel cell types are being developed and used, depending on the purpose of use and the fuel type or grade of purity available. The names of the different types mostly conform to the used electrolytes with direct-methanol-fuel cells being an exception.
Low-temperature fuel cells work at temperatures between 60°C and 120°C. Hydrogen and methanol act as a fuel. These fuel cells require a high degree of purity of the fuels and have an efficiency between 30% (methanol) and 45% (hydrogen). For the membrane that separates the two gases synthetic materials are used, which is why they are called polymer-electrolyte fuel cells (PEFC).
While PEFCs are operated with gaseous fuels, the direct methanol fuel cells (DMFC) uses an aqueous methanol solution.
High-temperature fuel cells work at temperatures between 550°C and 1100°C. This has the advantage that the fuel's degree of purity needs to be considerably lower and, at the same time, methane and carbon monoxide can be used as fuels directly. The electrical efficiency lies between 50% - 55% and, in conjunction with a downstream gas turbine that uses the exhaust fumes, can go up to 65% for the entire process. This is the best value for efficient power production.
High-temperature fuel cells are made from ceramics with either ceramic or metal connection plates. One can distinguish between solid oxide fuel cells (SOFC) and molten carbonate fuel cells (MCFC).
Continue with Grid Access